Draw in Unit Cubes the Crystal Planes
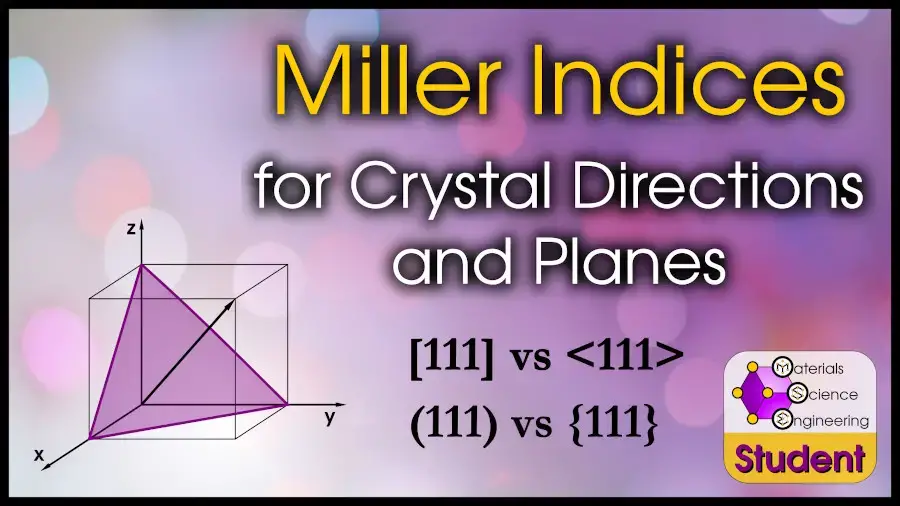
Miller indices are one of the most non-intuitive concepts most people encounter in an introductory course. And, since a few annotation differences can completely modify the meaning, advanced students too come back to review Miller Indices.
In this article, I'll explain what Miller indices are, why they're important, and how you can read and write them. Avant-garde students can skip straight to the Review if they're just looking for a quick refresher.
Miller Indices are a iii-dimensional coordinate system for crystals, based on the unit jail cell. This coordinate arrangement can indicate directions or planes, and are often written equally (hkl). Some common examples of Miller Indices on a cube include [111], the body diagonal; [110], the face up diagonal; and (100), the face plane.
By the time you end this article, you'll know what those numbers and symbols mean!
Basic Notation
The start supposition of Miller Indices is that you know the crystal family unit. If y'all don't know what crystals are, this topic will exist super disruptive–you lot might want to check out this article outset. If you're not certain well-nigh the different crystal families, y'all can read an explanation in this article about Bravais Lattices, merely as long every bit you know what a "cube" is, you can understand Miller Indices.
Every crystal can be depicted as a hexahedron (that means it has 6 faces, like a cube). There are some crystallographic coordinate systems which take "extra" dimensions, like the (hkil) Miller-Bravais system for hexagonal crystals, simply yous can ever reduce a conventional crystal cell into a primitive jail cell which is easily described by the (hkl) Miller Indices.
This commodity volition continue with the traditional Miller Indices.
Miller Indices are a coordinate organisation (like the cartesian or polar coordinate systems you learned in loftier school), so the first affair you need is an origin.
The origin is the bespeak ![]() and you can define information technology anywhere in your crystal. In nearly cases, the back left corner of the crystal is the nearly natural signal to define the origin.
and you can define information technology anywhere in your crystal. In nearly cases, the back left corner of the crystal is the nearly natural signal to define the origin.
You also need a sense of scale. For miller indices, the scale is the size of the unit of measurement cell. In other words, the value "0" is the origin of one unit of measurement cell, and the value "1" is the origin for the next unit cell over.
Miller indices also have weird way of writing negatives. Allegedly this was developed to save space in erstwhile crystallography journals. Instead of writing negative i as "-one," we write it as "![]() " and pronounce it as "bar 1." If you saw
" and pronounce it as "bar 1." If you saw ![]() you would pronounce that similar "the zero bar i zero management."
you would pronounce that similar "the zero bar i zero management."
It's also important to retrieve that crystals are defined past their symmetry. That's why the selection of origin is arbitrary. In some cases, we may want to distinguish between a specific direction, and all equivalent directions. We brand this stardom with brackets.
Don't worry, these general rules will make sense when we apply them to the specific case of points, directions, and planes.
Crystallographic Coordinate Arrangement
Miller indices apply a coordinate system which is very like to the cartesian coordinate system. The cartesian system is the regular second or 3D coordinate arrangement yous used in high school, which has 3 perpendicular axes x, y, and z.
If you want a review of the cartesian system, click to aggrandize.
Imagine you lot had a box with a length of 4, a width of ii, and a tiptop of iii. Put the lesser left corner of the box at the origin of your cartesian 3D system. What is the position of the elevation correct corner?
If you followed this picture show, you tin can see that the top right corner of the box is at the indicate ![]() . Its position is 4 spaces along the 10-centrality, ii spaces along the Y-axis, and iii spaces forth the Z-centrality.
. Its position is 4 spaces along the 10-centrality, ii spaces along the Y-axis, and iii spaces forth the Z-centrality.
By definition, the origin is at ![]() considering it is 0 spaces along each centrality.
considering it is 0 spaces along each centrality.
The only thing that changes between the crystallographic cartesian system and the version you learned in loftier school is the axes orientation.
In high school, you probably saw the 10-centrality travel to the right, the Y-axis travel upwards, and the Z-centrality wasn't shown, but travelled out of the page.
In crystallography, we use the Z-axis much more than in high school math. A clearer way to describe these axes is to have the X axis travel towards you (downward and left), the Y axis travel to the right, and the Z-axis travel upward.
So the point ![]() would be ane step toward y'all, 2 steps to the right, and 3 steps upwards (Not i pace right, ii steps up, and 3 steps out of the page).
would be ane step toward y'all, 2 steps to the right, and 3 steps upwards (Not i pace right, ii steps up, and 3 steps out of the page).
The terminal thing to remember about crystallographic coordinates is that the X-, Y-, and Z-axes may non exist perpendicular to each other. In the cartesian system, they are always perpendicular. In a cubic crystallographic organisation, they are also perpendicular, because the cubic lattice parameters are perpendicular.
All the same, not all crystals take perpendicular lattice parameters. For example, a hexagonal lattice has 2 lattice parameters that are 120º to each other, which are both perpendicular to the 3rd lattice parameter.
As you tin can run across, the betoken ![]() looks a bit different depending whether you have a cubic or hexagonal crystal structure. It'due south incommunicable to discuss Miller Indices without knowing the underlying crystal structure.
looks a bit different depending whether you have a cubic or hexagonal crystal structure. It'due south incommunicable to discuss Miller Indices without knowing the underlying crystal structure.
Miller Indices for Points
Technically, Miller Indices don't be for points–but materials scientists and crystallographers represent points with an unnamed notation organization that is very similar to Miller Indices, so I will explain that hither. The main notation is that yous use parentheses () and commas. The style you write them is exactly the manner yous write cartesian coordinates.
I've really used this note system earlier in the article, and I'm certain you understood, considering information technology's very intuitive. You lot just need to follow the basic rules–0 is at the origin, and 1 is the altitude of ane unit cell. We as well use the alphabetic character "h," k," and "l" to designate the 3 different lattice parameters.
For example, in the cubic system, the 3 lattice parameters accept the same length and are all perpendicular to each other (this is the definition of cubic). So, for all cubic crystals, "h" is the length of the cube's border in the x-direction. "grand" is the length of the cube's edge in the y-direction. "l" is the length of the cube in the z-direction.
The aforementioned rules apply fifty-fifty in noncubic cases, just since the vector's aren't perpendicular to each other, the terms "x-axis, y-axis, and z-axis" don't really brand sense.
The point ![]() will e'er exist the top right corner, opposite the origin. The betoken
will e'er exist the top right corner, opposite the origin. The betoken ![]() will always be the centre of the crystal.
will always be the centre of the crystal.
Remember, when describing points you write the point as (h, k, fifty). Yous as well apply negative signs, rather than the "bar" note. In other words, y'all write ![]() instead of
instead of ![]() .
.
Miller Indices for Directions
To describe a direction, all you demand to know is the point yous want to travel to, relative to the origin. For case, if you lot want to travel to the correct, the indicate directly to the right of the origin ![]() is
is ![]() . All yous need to do is accept this point and properly format it.
. All yous need to do is accept this point and properly format it.
If you remember, the format for directions is a foursquare bracket ![]() . If y'all wanted to talk about the family of directions, utilise angle brackets
. If y'all wanted to talk about the family of directions, utilise angle brackets ![]() .
.
So, to indicate the direction "right" in a cubic crystal you would write ![]() . The direction "left" would exist
. The direction "left" would exist ![]() .
.
The ![]() direction looks a flake different in the hexagonal system, but it's withal simply the length and direction of the 2nd lattice parameter. If you lot wanted to show a line to the "right" in a hexagonal system (depending on where we define the original axes), you would need to use a linear combination of two lattice parameters. In other words,
direction looks a flake different in the hexagonal system, but it's withal simply the length and direction of the 2nd lattice parameter. If you lot wanted to show a line to the "right" in a hexagonal system (depending on where we define the original axes), you would need to use a linear combination of two lattice parameters. In other words, ![]() .
.
Finally, it'south customary to reduce fractions. The "length" of the direction doesn't affair. If y'all wanted to indicate a management that travels ¼ up the ten-axis while going all the way across the y-axis, information technology's traditional to write that every bit ![]() instead of
instead of ![]() , by multiplying that latter version past four until everything is a whole number.
, by multiplying that latter version past four until everything is a whole number.
Miller Indices for Planes
Reading Miller indices for planes is a fleck different, considering nosotros have to enter "reciprocal space."
Reciprocal space means you take the inverse of whatever point you were thinking of. The inverse of i is nonetheless i, the changed of 2 is ½, and the inverse of 0 is infinity.
Here is the 3-step process to find the miller indices for planes.
- Discover the indicate where the plane intersects each centrality. If the aeroplane never intersects an axis considering it is parallel to that centrality, the intersection indicate is ∞.
- Accept the inverse of each intersection point.
- Put those 3 values in the proper (hkl) format. Recollect that negatives are expressed with a bar, parenthesis () bespeak a specific plane, and curly brackets {} bespeak the family of planes. Don't use whatsoever commas or spaces!
In a cubic system, information technology turns out that the direction ![]() will always be perpendicular to the plane
will always be perpendicular to the plane ![]() . For example the
. For example the ![]() direction is perpendicular to the
direction is perpendicular to the ![]() plane.
plane.
This is non necessarily truthful in not-cubic systems.
Directional Families
Directional families are the set of identical directions or planes. These families are identical considering of symmetry.
Imagine that I handed you a cube and asked you to draw the ![]() . By now, I hope you lot could exercise this easily! Yet, if I gave the same cube to someone else, they would probably describe a different
. By now, I hope you lot could exercise this easily! Yet, if I gave the same cube to someone else, they would probably describe a different ![]() , considering they chose a different origin or a different initial rotation.
, considering they chose a different origin or a different initial rotation.
The line you lot originally drew may wait like ![]() compared to the other person's version of
compared to the other person's version of ![]() .
.
In this way, we can say that ![]() and
and ![]() belong to the same directional family. The only way to distinguish between the two is to define a consequent rotational frame of reference. This means that any cloth belongings which is true along
belong to the same directional family. The only way to distinguish between the two is to define a consequent rotational frame of reference. This means that any cloth belongings which is true along ![]() will as well exist truthful of
will as well exist truthful of ![]() or any other management in the
or any other management in the ![]() family.
family.
To discover the different directional families, discover all the permutations that can replace ![]() with a negative version, such as
with a negative version, such as ![]() or
or ![]() . If the lattice vectors are the same length and have the aforementioned angle between them, yous can also change the order, such as
. If the lattice vectors are the same length and have the aforementioned angle between them, yous can also change the order, such as ![]() or
or ![]() .
.
Here is a list of the private directions in the directional families ![]() ,
, ![]() ,
, ![]() . If two directions belong to the same directional family unit, their corresponding planes volition likewise vest to the same planar family unit.
. If two directions belong to the same directional family unit, their corresponding planes volition likewise vest to the same planar family unit.
Since the cubic lattice has the nearly symmetry, in that location are the about number of identical directions in each directional family. Imagine, all the same, that you had a tetragonal crystal that was longer in the ![]() direction than the
direction than the ![]() direction. In this case, they would Non belong to the same family unit.
direction. In this case, they would Non belong to the same family unit. ![]() and
and ![]() would vest to the same family unit, which you lot could call
would vest to the same family unit, which you lot could call ![]() or
or ![]() . Nevertheless, the
. Nevertheless, the ![]() family would only include
family would only include ![]() and
and ![]() .
.
Identifying directional families becomes specially confusing if the lattice parameters h, k, and fifty are not perpendicular to each other. This was the master motivation for creating Miller-Bravais indices, which just apply to hexagonal crystals and convert the three-term (hkl) values into 4-term (hkil) values. This conversion is a bit complex, but allows yous to identify hexagonal directional families only based on the numerical value of the index.
Alternative Notations
Advanced topic, click to expand.
This is going into collapsable text because it's an advanced topic, near different letters that may exist used to designate different axes or positions along the axes.
In this commodity, I've tried to use h, k, and fifty, for the values inside the Miller Index, and x-centrality, y-axis, and z-axis for the directions.
It's also common to employ "U," "V," and "Due west" to designate directions, every bit in [UVW] vs (hkl).
Additionally, the mode I used x-, y- and z-axes is technically incorrect. We're technically supposed to employ the lattice vectors, rather than cartesian axes. In the cubic organisation, they are the aforementioned, simply they are not the same in other crystal systems.
Lattice vectors are often described using the letters " ![]() ," "
," " ![]() ," and "
," and " ![]() ." Sometimes you lot might run across the latters "
." Sometimes you lot might run across the latters " ![]() ," "
," " ![]() ," and "
," and " ![]() ," although this notation is typically used only with primitive cells.
," although this notation is typically used only with primitive cells.
I have tried to write this commodity in a way that is near understandable for people trying to acquire Miller Indices, but I retrieve it's important to know that you'll meet pocket-size notational differences in real scientific journals or textbooks that discuss some theory of the indices. In most practical cases, y'all volition just need to sympathise the meaning of basic indices such every bit ![]() ,
, ![]() ,
, ![]() , and
, and ![]() .
.
Review
At present you lot know how to read and write Miller indices! For a quick review of notation:
- (h, k, l) is for points. Remember to use the negative sign (-h) instead of bar sign (
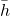 ) and don't reduce fractions–these rules apply to directions and planes.
) and don't reduce fractions–these rules apply to directions and planes. - [hkl] is for a specific management.
- <hkl> is for a family of directions.
- (hkl) is for a specific plane. Call up most reciprocal (inverse) space in planes!
- {hkl} is for a family of planes.
Earlier you go, you may be interested in practicing a few instance problems.
Case Problems
Practice 1. Draw the ![]() ,
, ![]() , and
, and ![]() directions in a cubic crystal.
directions in a cubic crystal.
Click here to bank check out the solution!
- Ascertain an origin. I'll cull the back left corner to define equally my
 signal
signal - Detect the respective
 ,
, 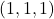 , and
, and 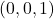 points because they have the aforementioned
points because they have the aforementioned 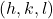 values as the directions y'all desire.
values as the directions y'all desire. - The line from the origin to these points, extending infinitely, is your direction.
Practice 2. Write the Miller Indices of the indicated direction.
Click here to check out the solution!
Beginning, define an origin. Information technology'south ever okay to motility the origin later on, since crystals-by definition–echo betwixt unit of measurement cells. In this case, you demand the origin to intersect forth the indicated direction, so you can move the origin the back left corner. Alternatively, you could simply interpret that vector then it intersects with the dorsum-left corner.
Either manner, you'll encounter that it takes a motion of 0 unit of measurement cells in the x-direction, 0 unit cells in the y-management, and 1 unit cell in the z-direction to move forth that vector. Thus, the direction is ![]() .
.
Practice three. Write the position of the signal, and the Miller Index for the management from the origin to the point. Assume the origin is at the back left corner.
Click here to check out the solution!
Hopefully it's straightforward to find this point, especially since I labelled the position for you. You demand to translate one unit jail cell in the ten-direction, ⅔ unit of measurement cell in the y-direction, and ½ unit cell in the z-direction. Thus, the signal is at location ![]() .
.
The direction would be identical, except that nosotros adopt to avoid fractions. Remember that directions extend infinitely, so we tin can easily multiply the ![]() value by 6, which is a common denominator. Thus, the direction is actually
value by 6, which is a common denominator. Thus, the direction is actually ![]() .
.
Practice 4. Draw the directions ![]() and
and ![]() , and write the Miller Index for the airplane mutual to both directions.
, and write the Miller Index for the airplane mutual to both directions.
Click here to bank check out the solution!
Hmm…What should yous practice about a negative value? Travel out of the unit of measurement cell? That'south a perfectly valid procedure, considering at that place is an identical unit cell behind the original version. If you notice, in that second unit jail cell it looks similar the vector comes out of the forepart-left corner.
Because there's always translational symmetry betwixt unit cells, you can freely ascertain a convenient origin (such as the forepart-left corner), change your frame of reference to a different unit cell, or simply translate the management to stay inside your current unit cell.
If we draw both directions like this, it's articulate that the plane between them is the "basal" plane, or the floor/roof (recollect by translation, the floor of ane unit cell is the roof of another).
To find the aeroplane, we need to make up one's mind where it intersects with the lattice parameters.
- This plane never intersects with the x-axis or y-centrality, considering it is parallel to them. Thus, the h value is ∞ and the k value is ∞. The plane intersects with the z-axis at point 0. Past translation, 0 is also 1, so the l value is 1.
- The reciprocal of ∞ and 1 is 0 and 1.
- Thus, the (hkl) value of the plane is (001).
- As a sanity check, recall that in cubic systems, the
![Rendered by QuickLaTeX.com [hkl]](https://msestudent.com/wp-content/ql-cache/quicklatex.com-f20d28e38053dbe599082cead70c0354_l3.png) management will exist perpendicular to the
management will exist perpendicular to the 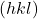 aeroplane. By now I promise it's easy to draw the
aeroplane. By now I promise it's easy to draw the ![Rendered by QuickLaTeX.com [001]](https://msestudent.com/wp-content/ql-cache/quicklatex.com-0b2a5b74f4c8f7ca8a907567d8ed65e0_l3.png) direction, which you lot can see is perpendicular to the
direction, which you lot can see is perpendicular to the 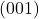 plane.
plane.
Practice 5. Draw the ![]() and
and ![]() planes in a cubic crystal.
planes in a cubic crystal.
Click here to check out the solution!
By the reciprocal dominion, the ![]() aeroplane intersects the x-centrality at ½, the y-centrality at ½, and never intersects the z-axis. The
aeroplane intersects the x-centrality at ½, the y-centrality at ½, and never intersects the z-axis. The ![]() plane intersects the 10-centrality at i, the y-axis at ane, and never intersects the z-axis. We can draw that like this.
plane intersects the 10-centrality at i, the y-axis at ane, and never intersects the z-axis. We can draw that like this.
Notice that the ![]() and
and ![]() are parallel, but not identical. If I had an atom at
are parallel, but not identical. If I had an atom at ![]() it would intersect the
it would intersect the ![]() plane but not
plane but not ![]() plane. This stardom matters more if you practice diffraction experiments.
plane. This stardom matters more if you practice diffraction experiments.
Practice 6. Draw the ![]() direction and
direction and ![]() plane in the hexagonal lattice.
plane in the hexagonal lattice.
Click here to check out the solution!
Let's beginning identify the direction. Remember, the hexagonal lattice parameters are not perpendicular, but I'll keep calling them the x-, y-, and z-axes because that is more familiar for most of my readers.
The ![]() is one step in the x-direction and one step in the y-direction, similar so:
is one step in the x-direction and one step in the y-direction, similar so:
To notice the plane, let'due south plot our intersection points. It volition intersect the x- and y-axes at the end of the unit cell (reciprocal of i is 1), and will be parallel to the z-centrality (reciprocal of 0 is infinity).
References and Further Reading
If you want to cheque your piece of work, you tin notice a "Miller Index plane calculator" for cubic lattice from the University of Cambridge Dissemination of It for the Promotion of Materials Science.
If you lot're reading this article as an introductory student in materials scientific discipline, welcome! I hope you lot can find many other useful articles on this website. You may be interested in a related article I've written about Atomic Packing Factor.
If you're reading this article because y'all're taking a class on structures, you may be interested in my other crystallography articles. Here is this list, in recommended reading order:
Introduction to Bravais Lattices
What is the Difference Betwixt "Crystal Structure" and "Bravais Lattice"
Atomic Packing Factor
How to Read Miller Indices
How to Read Hexagonal Miller-Bravais Indices
Shut-Packed Crystals and Stacking Order
Interstitial Sites
Primitive Cells
How to Read Crystallography Notation
What are Point Groups
List of Point Groups
If you are interested in more details about whatsoever specific crystal structure, I accept written private manufactures near elementary crystal structures which represent to each of the 14 Bravais lattices:
1. Simple Cubic
two. Face-Centered Cubic
2a. Diamond Cubic
3. Body-Centered Cubic
4. Simple Hexagonal
4a. Hexagonal Close-Packed
4b. Double Hexagonal Close-Packed (La-type)
v. Rhombohedral
5a. Rhombohedral Shut-Packed (Sm-blazon)
half-dozen. Uncomplicated Tetragonal
7. Torso-Centered Tetragonal
7a. Diamond Tetragonal (White Tin)
eight. Simple Orthorhombic
9. Base-Centered Orthorhombic
10. Face-Centered Orthorhombic
11. Body-Centered Orthorhombic
12. Simple Monoclinic
13. Base-Centered Monoclinic
fourteen. Triclinic
Source: https://msestudent.com/miller-indices/